The Science Behind Genomind
The science behind Genomind
Precision Medicine
Precision or “personalized medicine” is an innovative care model designed to optimize the efficiency and therapeutic benefits for patients through the integration of genetic, environmental, and lifestyle factors. Precision medicine often involves pharmacogenetic testing.
Pharmacogenetics
Pharmacogenomics is a field of study combining the science of pharmacology and genomics to reveal how an individual’s genetics may influence and metabolize certain medications or determine the potential impact medications can have on the body.

Medication Management
A process aimed at optimizing patient outcomes by monitoring, reconciling, and at times, adjusting medications combinations. Genomind’s solutions bring the added layer of genetics to the existing tools used by providers.
Built on the most up-to-date clinical evidence
Our medical affairs team holds monthly literature reviews to ensure the evidence behind our PGx report is current. Below are several key sources for pharmacogenetic guidance.

The U.S. Food and Drug Administration (FDA) Table of Pharmacogenetic Associations and Table of Pharmacogenetic Biomarkers in Drug Labeling list 250+ drugs with pharmacogenetic precautions, warnings or dosing guidance. The FDA includes gene-drug associations in many mandatory product labels or package inserts (PI).
Recognized internationally, the Clinical Pharmacogenetics Implementation Consortium (CPIC) standardizes the field of pharmacogenetics, including terminology and genotype to phenotype conversion tables. The group curates peer-reviewed, evidence-based, and detailed gene/drug clinical practice guidelines.


The Dutch pharmacist trade organization KNMP founded DPWG (Dutch PGx Working Group) – a European consortium whose objective is to translate PGx research into therapeutic (dose) recommendations.
The Pharmacogenetics Knowledge Base (PharmGKB) is a comprehensive database funded by the National Institute of Health (NIH), which summarizes gene-drug association guidelines and resources compiled across government agencies and expert groups like the FDA, CPIC and DPWG.

Summary of clinical studies
Note: The ‘Genecept Assay®’ mentioned several times below was an earlier version of what is now Genomind® Pharmacogenetic (PGx) Report.
Pharmacogenetic Testing Among Patients With Mood and Anxiety Disorders Is Associated With Decreased Utilization and Cost: A Propensity-Score Matched Study
Citation: Perlis RH, et al. Pharmacogenetic testing among patients with mood and anxiety disorders is associated with decreased utilization and cost: A propensity-score matched study. Depression & Anxiety. 2018;35(10):946-952. doi:10.1002/da.22742
Study design: Case-control study examining health care utilization and cost among patients with mood disorders following use of Genomind PGx testing (n=817) compared to a matched control group (n=2,745) whose treatment was not guided by Genomind PGx.
Genomind PGx testing was associated with:
Randomized, Controlled, Participant‐ and Rater‐blind Trial of Pharmacogenomic Test‐guided Treatment versus Treatment as Usual for Major Depressive Disorder
Citation: Perlis RH, et al. Randomized, controlled, participant- and rater-blind trial of pharmacogenomic test-guided treatment versus treatment as usual for major depressive disorder. Depress Anxiety. 2020;37(9):834-841. doi:10.1002/da.23029
Study design: Eight-week multicenter RCT examining the impact of Genomind PGx testing (n=151) versus treatment-as‐usual (n=153) among outpatients with major depressive disorder. Both participants and raters were blinded to treatment conditions for the primary outcome (Hamilton Depression Rating Scale; SIGH‐D‐17).
There were no significant differences between groups on the primary outcome measure (change in SIGH-D-17); however, exploratory analyses suggested:
A Naturalistic Study of the Effectiveness of Pharmacogenetic Testing to Guide Treatment in Psychiatric Patients With Mood and Anxiety Disorders
Citation: Brennan FX, et al. A naturalistic study of the effectiveness of pharmacogenetic testing to guide treatment in psychiatric patients with mood and anxiety disorders. Prim Care Companion CNS Discord. 2015;17(2).doi:10.4088/PCC.14m01717
Study design: Naturalistic, open-label study of psychiatric patients who used Genomind PGx testing (n=685) and completed self-report questionnaires assessing depression (Quick Inventory for Depressive Symptoms), anxiety (Zung Self-Rated Anxiety Scale), and quality of life (Quality of Life Enjoyment and Satisfaction Questionnaire – Short Form).
87% of participants showed clinically measurable mental health improvement. Results demonstrated a substantial proportion of individuals receiving Genomind PGx testing showed:
Psychiatric Pharmacogenetic Testing in an Adult Psychiatric Inpatient Population
Citation: King CD, et al. Open-label pilot study of psychiatric pharmacogenetic testing in an adult psychiatric inpatient population. Elsevier. 2020. doi:10.1016/j.pmip.2020.100060
Study design: Open-label pilot study examining the feasibility of Genomind PGx testing in an inpatient unit, examining clinical outcomes including the APA DSM-V Level 1 Cross Cutting Symptom Measure, APA DSM-V Level 2 Cross Cutting Symptom Specific Measure (8 specific symptoms), and the WHODAS 2.0 to assess quality of life, 3 months post-hospitalization in patients with anxiety and depression related diagnoses
Compared to a control group who did not have PGx testing, participants who received Genomind PGx testing reported:
Predicting potential drug-drug-gene interactions in a population of individuals utilizing a community-based pharmacy
Citation: Dowd D, et al. Predicting potential drug-drug-gene interactions in a population of individuals utilizing a community-based pharmacy. Presented at: Neuroscience Education Institute Congress; November 3-7, 2021; Colorado Springs, CO.
Study design: De-identified prescription data from March 2020 provided by four Express RX pharmacies (Arkansas, USA) for 4,761 individuals was used to quantify and describe potential drug-drug, drug-gene, and drug-drug-gene interactions in a community-based patient population.
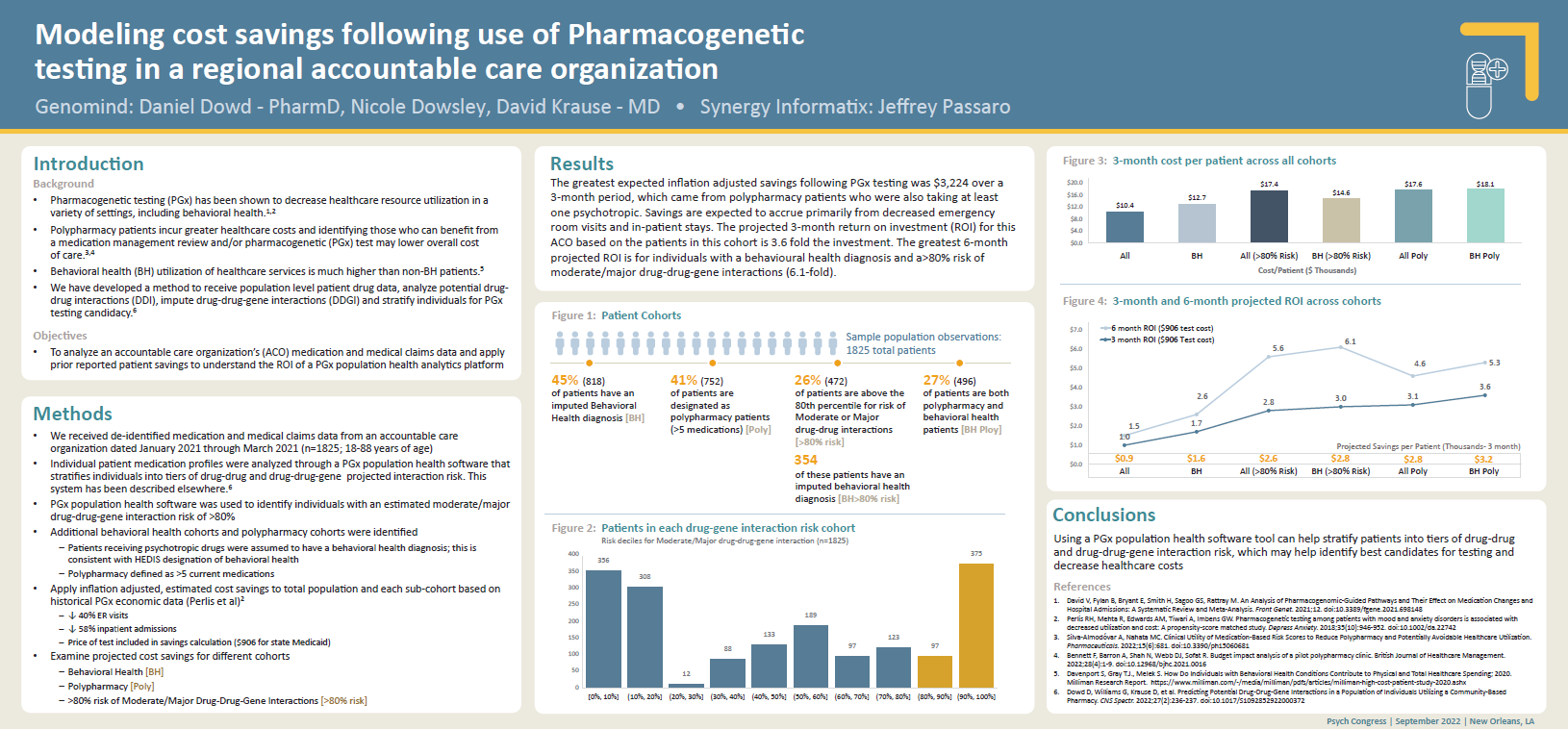
Modeling cost savings following use of pharmacogenetic testing in a regional accountable care organization
Citation: Dowd D, et al. Modeling Cost Savings Following Use of Pharmacogenetic Testing in a Regional Accountable Care Organization. Presented at: Psych Congress; September 17-20, 2022; New Orleans, LA. Study design: De-identified prescription and medical claims data from January-March 2021 was provided by an accountable care organization for 1,825 individuals. Data was used to quantify drug-drug-gene interaction risk and cost savings in patient tiers.Pharmacogenetic-guided psychiatric intervention associated with increased adherence and cost savings
Citation: Fagerness J, et al. Pharmacogenetic-guided psychiatric intervention associated with increased adherence and cost savings. Am J Manag Care. 2014;20(5):e146-56.
Study design: Retrospective study utilizing medical claims databases of U.S. patients covered by commercial health insurance, Medicare, and Medicaid. Patients with a psychiatric diagnosis, treatment, and use of the Genomind Genecept Assay were compared with age and disease severity-matched controls with no use of the Assay.
Genomind Genecept Assay-guided treatment was associated with:
Medication Optimization Using Pharmacogenomic Testing and a Drug Interaction Guide in a Complex Mental Health Population
Citation: Wood, et al. Veterans Affairs MedOpt Polypharmacy Study. Presented at: Psych Congress; October 3-6, 2019; San Diego, CA.
Study design: 12-week pilot study examined the clinical impact of provider access to PGx results and Genomind’s GDIG tool in Veterans prescribed polypharmacy, defined as 5 or more medications with at least 2 for a mental health indication. Psychiatric medication providers were given access to the information, but were allowed to make their own decisions regarding medication management. Veteran outpatients (N=53) prescribed polypharmacy (Mean=13.15 medications) were enrolled into the study.
Clinical utility of pharmacogenetics-guided treatment of depression and anxiety
Citation: Boland JR, et al. Clinical utility of pharmacogenetics-guided treatment of depression and anxiety. Personalized Medicine in Psychiatry. 2017;7:7-13. doi:10.1016/j.pmip.2017.11.001
Study design: Follow-up analysis of open-label study (Brennan 2015) referenced above, looking at subset of participants with variants of SLC6A4 and MTHFR . Comparison of outcomes with Genomind Genecept assay-guided treatment with those of treatment discordant with the assay.
Individuals whose subsequent treatment was congruent with the assay-guided treatment per the Genomind Genecept Assay reported:
Summary of external meta-analyses for PGx
Multiple category data analyses support improved outcomes with pharmacogenomic testing.
The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: A meta-analysis
Citation: Rosenblat JD, et al. The effect of pharmacogenomic testing on response and remission rates in the acute treatment of major depressive disorder: a meta-analysis. J Affect Disord. 2018;241:484-491. doi:10.1016/j.jad.2018.08.056.
Study design: Rosenblat and colleagues meta-analytic review examined the effect of PGx testing on remission and response rates specifically in the acute treatment phase of MDD on a total of six studies: four RCTs (also included in the Bousman meta-analysis), as well as two additional open-label, controlled cohort studies. A total of 735 participants were randomized to receive PGx-guided treatment (n=353) versus treatment as usual (n=383).
- Overall, the PGx-guided treatment participants had a remission rate of 40% as compared to the unguided group, with a pooled remission rate of 25%.
- In a random-effects meta-analysis examining 735 patients undergoing acute treatment for MDD across the included studies, the pooled risk ratio favored PGx-guided treatment, indicating a 74% increased odds of remission.
Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials
Citation: Bousman CA, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37-47. doi:10.2217/pgs-2018-0142
Study design: Bousman and colleagues performed a systematic review and meta-analysis examining five randomized controlled trials (RCTs) of the utility of PGx testing in major depressive disorder (MDD) patients (n=1,737). In each individual RCT included in the meta-analysis, patients were randomized to receive PGx-guided treatment (n=887) versus treatment as usual (n=850).
Depressed patients receiving PGx-guided treatment were 71% more likely to achieve remission on their medications compared to participants receiving treatment as usual.
An Analysis of Pharmacogenomic-Guided Pathways and their Effect on Medication Changes and Hospital Admissions: A systematic review and meta-analysis
Citation: David V, et al. An analysis of pharmacogenomic-guided pathways and their effect on medication changes and hospital admissions: a systematic review and meta-analysis. Front. Genet. 2021;12:698148. doi: 10.3389/fgene.2021.698148
Study design: Systemic review and meta-analysis examining the effect of PGx testing on medication changes and hospitalizations compared to treatment-as-usual (TAU).
- In the analysis of 5 studies evaluating hospitalizations, participants receiving PGx-guided treatment (n=2,957) were 50% less likely to be hospitalized compared to participants receiving treatment-as-usual (n=6,783).
- In the analysis of 5 studies evaluating medication changes, participants receiving PGx-guided treatment (n=749) were 91% more likely to have medication changes compared to TAU participants (n=825). Medication changes were a result of medication optimization (ex. medication switch, change of dose, or deprescribing).
Top certified laboratories
tested twice
to ensure a
99.9%
lab accuracy.
 (
(