Understanding the dynamic physiological changes that occur in the pediatric population will help to identify optimal drug dosing. The various factors that impact adult pharmacokinetics mature at different rates, thus requiring awareness of drug dose regimens in neonates, infants, and children.3
The FDA’s Center for Drug Evaluation and Research generally divides the pediatric population into the following groups by age:2
- Neonates: Birth up to 1 month
- Infants: 1 month up to 2 years
- Children: 2 up to 12 years
- Adolescents: 12 years up to 16 years
How age-related changes impact metabolism
Drug metabolism is influenced by several factors including pharmacogenetics, environment, and development. Age-related changes in metabolism can impact:
- Drug clearance
- Half-life
- Area under the curve (AUC)
- Maximum concentration (Cmax)
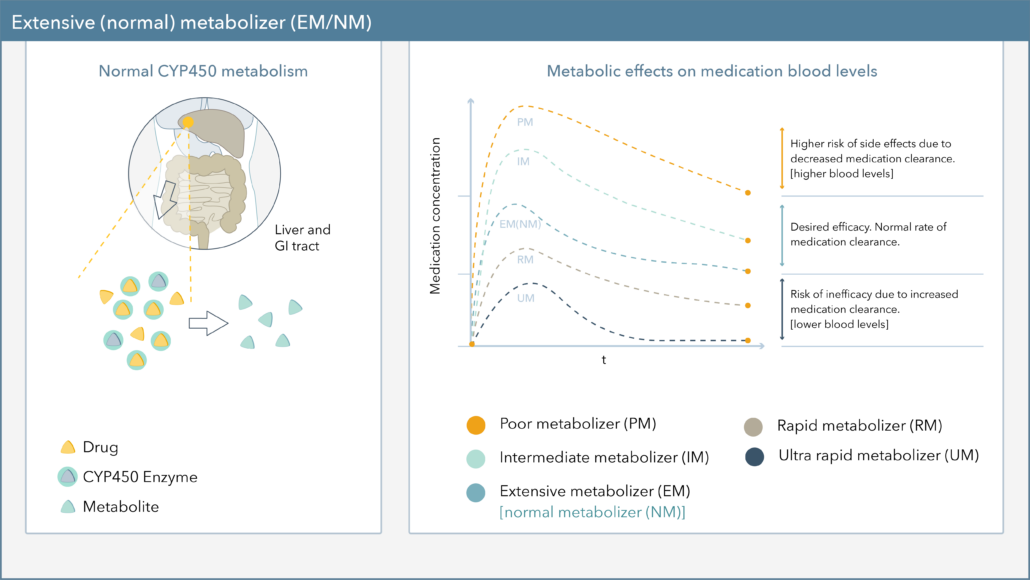
The major metabolic pathway and rate of metabolite formation can differ in pediatric patients compared to adults and differ even within the pediatric population. Each drug metabolizing enzyme system (Phase 1 and 2) appears to have its own unique pattern of age-dependent development. As shown in Figure 1, the cytochrome P450 (CYP) enzymes are expressed at differing levels during development (Figure A). 1-5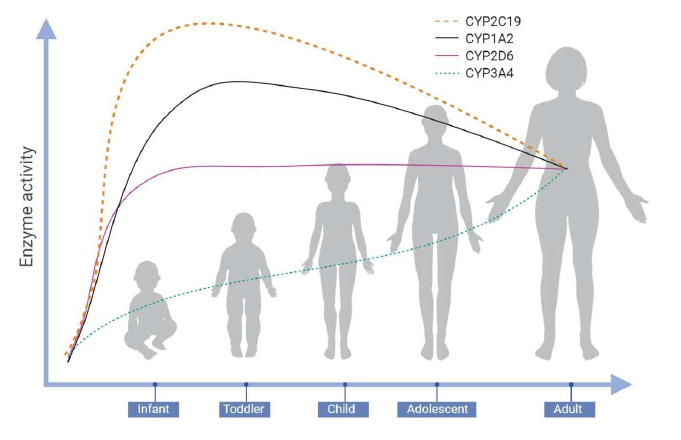
Figure A
For example, consider the benzodiazepine Valium (diazepam). Diazepam is primarily metabolized by CYP2C19 and CYP3A4 to pharmacologically active metabolites which are then further metabolized and largely eliminated by glucuronidation (Figure B).7
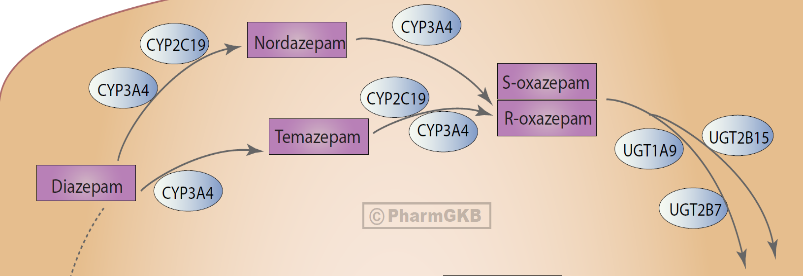
Figure B
In both premature and full term infants, the active metabolite shows evidence of continued accumulation as compared to children. The longer half-lives in infants may be a result of incomplete metabolic pathway maturation. It is important to recognize that diazepam
accumulates upon multiple dosing which may prolong the terminal elimination half-life.6
How developmental changes impact pharmacokinetic measures
Growth and developmental changes in the pediatric population can have a profound effect on a medication by influencing metabolism as well as several other key pharmacokinetic measures.
- Oral drug absorption can be affected by the differences in factors such as gastric acidity and GI transit time.
- Changes in skin, muscle, and fat can affect intramuscular, subcutaneous, or percutaneous drug absorption.
- Drug distribution can be affected by changes in body composition, blood flow to an organ, plasma protein binding, and tissue binding.
- Excretory pathways (kidney, biliary, and pulmonary) mature at different rates and are also subject to age-related differences.
Although developmental changes are recognized, information on drug metabolism of specific drugs in newborns, infants, and children is limited.1-4
In conclusion
When prescribing for children and adolescents, it is important to consider these developmental changes. Predicting a child’s likelihood of tolerating and responding to a psychotropic medication has proved challenging and has opened the door for pharmacogenetic guidance.
PGx testing can assist clinicians in the selection of safe and appropriate treatments and is intended to be used adjunctively to a complete patient assessment. Important advancements have been made in the application of PGx testing in psychiatry in recent years and the PGx knowledge base in pediatric populations is emerging.8
Deliver targeted and personalized care with Genomind.
Register with Genomind to use our precision tools and services and help your patients get better.
References
- Mangoni, A. A., & Jackson, S. H. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. British journal of clinical pharmacology, (2004). 57(1), 6–14.
- U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). FDA Draft guidance for industry: general clinical pharmacology: considerations for pediatric studies for drugs and biological products. 2014
- Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther. 2014;19(4):262-276. doi:10.5863/1551-6776-19.4.262
- Fernandez, E., et al. Factors and Mechanisms for Pharmacokinetic Differences between Pediatric Population and Adults. Pharmaceutics. 2011;3(1):53-72.
- Ramsey, L. B., et al. Thoughtful Clinical Use of Pharmacogenetics in Child and Adolescent Psychopharmacology. Journal of the American Academy of Child and Adolescent Psychiatry, (2020) S0890-8567(20)31357-5.
- Valium. Package Insert. Roche; 2016
- Whirl-Carrillo, M., et al. Pharmacogenomics Knowledge for Personalized Medicine. Clinical Pharmacology & Therapeutics (2012). 92(4): 414-417.
- Namerow, L.B., Walker, S.A., Loftus, M. et al. Pharmacogenomics: an Update for Child and Adolescent Psychiatry. Curr Psychiatry Rep. 2020; Rep 22, 26.