The term pharmacokinetics has historically been defined by the phrase “what the body does to a drug.” In greater detail, the science of pharmacokinetics describes a drug’s journey throughout the body and focuses on the absorption, distribution, metabolism, and excretion of drugs.1
What is drug metabolism?
One of the major components of pharmacokinetics is drug metabolism, which leads to the biotransformation of drugs within the body.
This chemical modification of drugs has been defined to occur in three phases.
- Phase I metabolism includes oxidation, reduction, and hydrolysis reactions, and leads to the production of polar, water-soluble metabolites.
- Phase II metabolism leads to the formation of larger, water-soluble, inactive compounds that are more easily excreted.
- Phase III metabolism during which the drug may undergo further transformation and excretion.2-4
How do CYP450 enzymes play a crucial role in drug metabolism?
Within Phase I metabolism, the CYP450 family of enzymes play a major role. These enzymes are primarily located in the liver, but can also be found throughout other areas of the body, and have been characterized as the main enzyme system for drug metabolism; in fact, 70-80% of medications are metabolized by the CYP450 system.5-6
What CYP450 genetic variants does Genomind report?
The Genomind pharmacogenetic report outlines genetic variants in six key CYP450 genes: 2D6, 2C9, 2C19, 2B6, 3A4/3A5, and 1A2. Within the reporting of these genes, one will notice the star (*) allele nomenclature.
This naming system was developed as a means of standardizing variants within the CYP450 genes 7, and each star allele is associated with a functionality (e.g. normal function, increased function, decreased function, no function, etc.).
The combination of star alleles and the associated functionalities an individual expresses will then define their phenotype, or in other words, their metabolism status.
The Clinical Pharmacogenetics Implementation Consortium (CPIC) is the international association that has put forth standard definitions for CYP450 genotypes and associated phenotypes.8
Let’s look at a specific example of this:

As seen here, this patient has a *4 allele and a *9 allele for the CYP2D6 gene. According to CPIC, the *4 allele is associated with no enzyme function, whereas the *9 allele is associated with decreased enzyme function. When these two alleles are present together, the resulting phenotype would be intermediate metabolism within the CYP2D6 enzyme pathway.
How do CYP450 genetic variations affect drug metabolism?
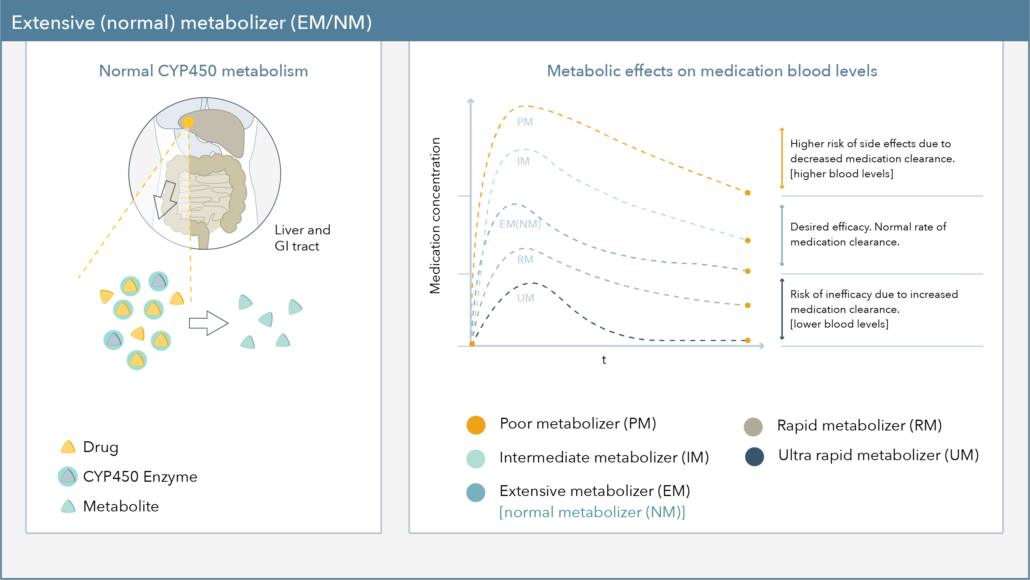
Taking a more in-depth look into metabolism status, Figure 1 demonstrates the effect genetic variations can have on CYP450 metabolism. Patients are typically reported as extensive/normal (EM/NM), intermediate (IM), poor (PM), rapid (RM), or ultra-rapid metabolizers (UM).9
- RMs and UMs, or fast metabolizers, are at risk for hyper-metabolism of medications, reduced blood levels, and may require a higher dose of medication to achieve therapeutic efficacy.
- IMs and PMs, on the other hand, are at risk for slower drug metabolism, increased blood levels, and may require a reduced dose of medication to decrease the risk for side effects.10
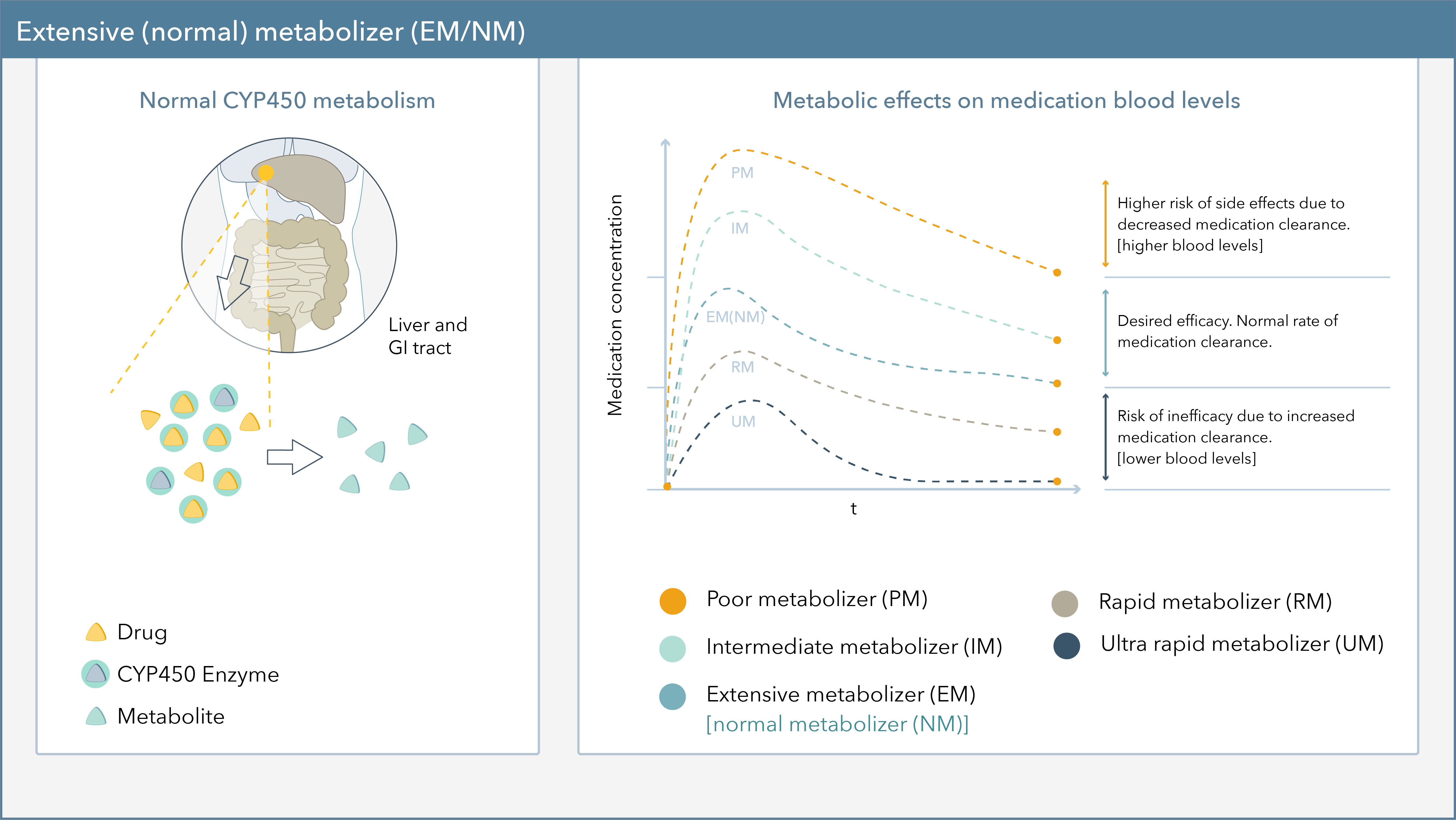 Figure 1. Metabolic Effects on Medication Blood Levels
Figure 1. Metabolic Effects on Medication Blood Levels
Several psychotropic medications have specific guidance regarding the clinical effects of CYP variants.
As an example, the FDA labeling for citalopram specifically cites “…in CYP2C19 poor metabolizers, citalopram steady state Cmax and AUC was increased by 68% and 107%, respectively. Citalopram [Celexa] 20mg/day is the maximum recommended dose in CYP2C19 poor metabolizers due to the risk of QT prolongation…”11 Outside of information provided by the FDA, additional clinical guidance for CYP variants is provided by CPIC and The Dutch Pharmacogenetics Working Group (DPWG).
Other ways CYP450 genetic variations impact individual drug metabolism
In addition to the impact CYP450 enzymes have on the metabolism of individual drugs, this enzyme family is also an important consideration in drug-drug interactions. Various medications can act as inhibitors or inducers of CYP450 enzymes, leading to alterations in the activity of these enzymes and their ability to metabolize other medications.3 This, in turn, can lead to increased or decreased concentrations of other medications, which may be associated with toxicities or changes in efficacy, respectively.
In Conclusion
In order to help clinicians navigate the CYP450 enzymes (as well as other pharmacokinetic considerations), Genomind provides clinicians with access to GenMedPro™, our gene-drug interaction software.
GenMedPro™ serves as an outstanding tool to help evaluate both gene-drug and drug-drug interactions, as well as alternative medication options, as appropriate. This robust interaction database can facilitate clinicians in determining personalized medication options and regimens for their patients. To learn more about GenMedPro™ or to request a demonstration from our PhD and PharmD experts, please register to order with Genomind.
References
- Zhao M, Wang Y, Chen M, Wu B. Introduction to Pharmacokinetics. In: Circadian Pharmacokinetics. Wu B, Lu, D, Dong D., eds. Singapore: Springer Singapore;2020.
- Susa ST, Preuss CV. Drug Metabolism. [Updated 2020 Nov 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. Available from: Drug Metabolism – StatPearls – NCBI Bookshelf (nih.gov)
- Phang-Lyn S, Llerena VA. Biochemistry, Biotransformation. [Updated 2020 Sep 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. Available from: Biochemistry, Biotransformation – StatPearls – NCBI Bookshelf (nih.gov)
- de Leon J. Gulcuronidation enzymes, genes and psychiatry. Int J Neuropsychopharmacol 2003;6(1):57-72.
- Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician 2007;76(3):391-396.
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013;138(1):103-41.
- Robarge JD, Li L, Desta Z, Nguyen A, Flockhart DA. The star-allele nomenclature: retooling for translational genomics. Clin Pharmacol Ther 2007;82(3):244-8.
- CPIC (Clinical Pharmacogenetics Implementation Consortium). Alleles. Available at: https://cpicpgx.org/alleles/
- Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. [Updated 2020 May 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. Available from: Biochemistry, Cytochrome P450 – StatPearls – NCBI Bookshelf (nih.gov)
- American Medical Association. Pharmacogenomics: Increasing the safety and effectiveness of drug therapy. Available at: https://crediblemeds.org/files/3913/6973/9557/pgx-brochure2011.pdf
- Celexa. Package insert. Forest Laboratories, Inc.; 2011