Psychotropic medications have various mechanisms of action but ultimately target just a few sites of action: G-protein-coupled receptors (GPCRs), neurotransmitter transporters, ion channels, and enzymes.
What are G-protein-coupled receptors (GPCRs)?
GPCRs are the largest family of membrane proteins and are characterized by the presence of seven transmembrane regions, which may contain one or more neurotransmitter binding sites. The G-protein receptor system interacts with many neurotransmitters and is essential to the signal transduction cascade as it influences the degree of downstream chemical activity. As a simplified overview, signal transduction cascades begin with a first messenger binding to a unique receptor, leading to activation of a different downstream second, third, and subsequent chemical messengers.
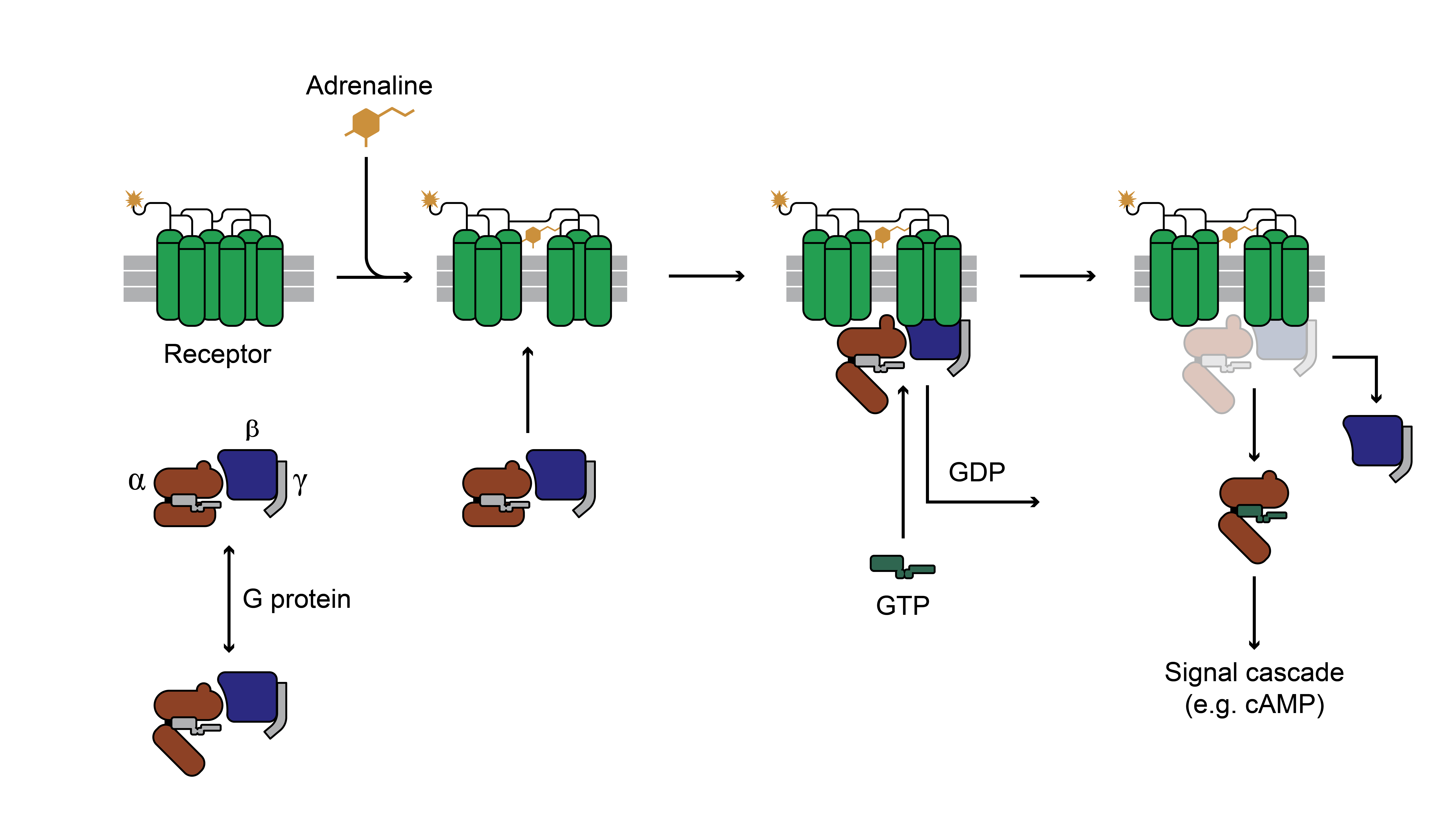
Adapted from the Proceedings of the National Academy of Sciences of the United States of America
For GPCRs, the first messenger is a neurotransmitter or drug that binds to its receptor and causes a conformational change so that the receptor-neurotransmitter complex can now activate or release the G-protein attached to the intracellular part of the protein.
The G-protein, once activated, will separate into two unique molecules. These molecules can then bind to other protein targets within the cell that are capable of synthesizing the second messenger, which can then go on to interact with other intracellular targets (i.e., continuing this cascade to third messengers and beyond). This neurotransmitter-induced molecular cascade will ultimately modify gene expression and be translated into diverse biological actions.
As discussed in the Monoamine Neurotransmitters and Psychopharmacology article, neurotransmission is the foundation of psychopharmacology. It is imperative to recognize and understand the GPCR system as it is essential to signal transduction and is the target of nearly one third of psychotropic medications. Many psychotropics act on the GPCR neurotransmitter binding sites or at allosteric sites on the receptor to modify the downstream molecular events and (hopefully) alleviate psychiatric symptoms.
Drugs can modify the actions of the GPCR in a number of ways that are summarized below:
- Agonists: Produce a conformational change in the GPCR that leads to receptor activation.
-
-
- Full Agonist: stimulates receptor to the greatest extent possible.
- Partial Agonist: stimulates receptor to a lesser degree than the natural neurotransmitter.
-
- Antagonists: Prevent the actions of agonists but do not have activity in the absence of the agonist. True antagonists can be considered “neutral” or “silent” since they have no actions of their own.
- Inverse Agonists: Block agonists and also can reduce activity of the receptor below the baseline level when no agonist is present.
Why Gene Mutations Affect Drug-Response, Side Effect Rate or Drug Exposure
While we’ve discussed several genes that code for receptors, transporters, and enzymes on the Genomind PGx test, we haven’t really explored why mutations in these genes may affect drug response, side effect rate, or drug exposure.
Depending on where the mutation lies within the DNA, it could produce changes including, but not limited to, a coded protein’s expression, function, mRNA stability, or localization (SNPs: impact on gene function and phenotype).
The genetic polymorphism may also be one part of a haplotype (i.e., several polymorphisms inherited together) in which each mutation plays a small role in drug response and exposure, but when looked at in combination, produce a stronger association that may be more predictive of clinical outcomes (e.g., CYP450 star allele system and drug exposure). It’s also possible that the genetic mutation associated with response or side effect rate is simply in linkage disequilibrium (i.e., non-random association of polymorphisms at different locations within the DNA) with the real culprit responsible for changes in drug response.
In order to simplify interpretation of these complex processes, this section of the Genomind 360 Mental Health Learning Center will focus on the effects of genetic polymorphisms associated with changes in expression and function of G-protein-coupled receptors.
Example: The A118G Polymorphism and Opioid Dose Requirements
OPRM1 is a gene that encodes for the μ-opioid receptor (MOR). The A118G polymorphism (rs1799971) is associated with increased opioid dose requirements. One explanation for why this may occur is that this polymorphism is associated with a change in the receptor itself.
The change from an adenosine to a guanine in the genetic code produces a change in this G-protein-coupled receptor (asparagine replaced by an aspartic acid). This change has been associated with reduced expression of the mRNA and protein in preclinical rodent models, as well as reduced binding potential of opioids in human patients (Ray, 2011; Weerts, 2013). Decreased binding potential of drugs like morphine and carfentanil may decrease opioidergic signaling, as discussed earlier in this chapter, and reduce the overall analgesic response to these medications.
Are You Ready to Upgrade Your Practice with Genomind?
Genomind’s pharmacogenetic testing is the most advanced and comprehensive mental health pharmacogenetic test available. Get access to 26 genes related to mental health, 130+ medications, 10+ conditions, state-of-the-art tools, and 360 degrees of support today! Get started here.
References
- Alhadeff R, Vorobyov I, Yoon HW, Warshel. Proceedings of the National Academy of Sciences Oct 2018, 115 (41) 10327-10332; DOI: 10.1073/pnas.1810316115
- Ray, Riju et al. “Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers.” Proceedings of the National Academy of Sciences of the United States of America vol. 108,22 (2011): 9268-73. doi:10.1073/pnas.1018699108
- Stahl, S. M. (2013). Transporters, receptors, and enzymes as targets of psychopharmacological drug action. In Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge, United Kingdom: Cambridge University Press.
- Weerts, Elise M et al. “Influence of OPRM1 Asn40Asp variant (A118G) on [11C]carfentanil binding potential: preliminary findings in human subjects.” The international journal of neuropsychopharmacology vol. 16,1 (2013): 47-53. doi:10.1017/S146114571200017X