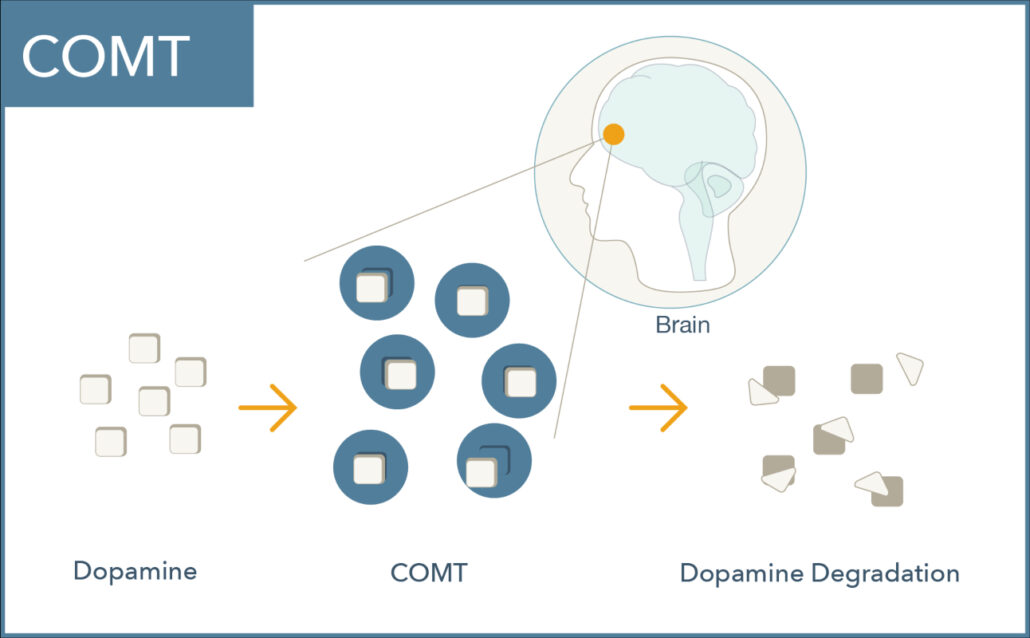
What does the COMT enzyme do?
COMT (catechol-O-methyltransferase) is a central enzyme that breaks down catecholamines including dopamine, norepinephrine, and epinephrine.
COMT plays a vital role in regulating dopamine signaling in the prefrontal cortex where dopamine transporters are relatively sparse.1 Dopamine levels here are critical for memory, attention, judgment, and other executive functions.2,3
COMT gene mutations and dopamine
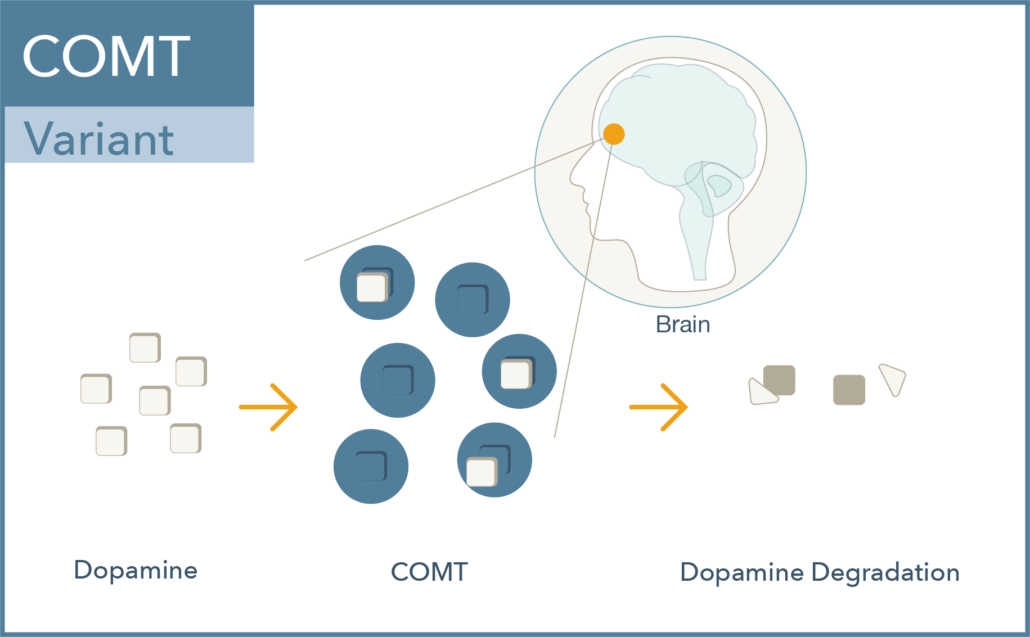
Val158met (rs4680) is a common single nucleotide polymorphism in the COMT gene which results in a change of one amino acid in the protein from a valine (Val) to a methionine (Met). The met allele is associated with approximately 40% lower enzymatic activity, leading to increased dopamine activity. Interestingly:
- The heterozygous genotype (Val/Met) is considered as the normal level of dopamine degradation or clinically non-actionable.
- Individuals with the Val/Val genotype display elevated COMT enzyme activity and thus reduced dopamine levels.
- Individuals with the Met/Met genotype have reduced COMT enzyme activity and thus elevated dopamine levels. 1,4-7
What is the clinical significance of COMT gene mutations?
Clinical studies have shown that the COMT Val/Val genotype may have behavioral consequences regarding cognitive function, memory, attention, motivation and judgment.7,8
In Val/Val (high-activity) patients, dopaminergic agents have been shown to improve executive function and working memory to a greater degree in both animal and human studies. However, these agents may produce a deleterious effect on cognition in Met/Met (low-activity) patients. Another class of drugs known as COMT inhibitors have also been shown to produce this biphasic effect on cognition in Val/Val versus Met/Met individuals.9-13
COMT and schizophrenia
Recent clinical studies investigating the effects of antipsychotic medications on cognitive function in schizophrenia and bipolar disorder found that patients with the Met/Met genotype had improved scores on measures of executive function (as well as positive symptoms of schizophrenia) when compared with their Val/Met and Val/Val counterparts.14-20
COMT and depression treatment
Alternative antidepressant therapeutic strategies include electroconvulsive therapy (ECT), transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS), which may be modulated by COMT genotype.
Studies have shown an association with COMT Val/Val genotype with greater sensitivity to ECT and improvements in depressive scores.21 There is also evidence COMT genotype may differentially impact executive function in tDCS therapy.22-24 TMS, similar to other neurostimulation techniques, has been shown to increase dopamine in the prefrontal cortex,25-30 but data evaluating the effect of COMT on TMS response has been limited.
COMT pharmacogenomics
Genomind’s pharmacogenetic testing focuses on COMT as it relates to dopamine in the prefrontal cortex (PFC) because there is a relative lack of dopamine transporter proteins in the PFC.31 The activity of these transporters can be up or down-regulated to balance the activity of enzymes. The relative lack of dopamine transporters in the PFC means that dopamine levels in the PFC are largely determined by activity of the COMT enzyme.
If clinically indicated, patients that display the COMT Val/Val genotype may benefit from agents that increase synaptic dopamine. For example, Val/Val patients showed improved cognition in response to amphetamine while Met/Met patients did not.10
Met/Met patients have higher than average dopamine levels, and a number of studies indicate that those patients tend to have superior executive function skills as compared to Val/Met and Val/Val patients.7 Met/Met patients may show impairments in cognition in response to dopamine agonists, since synaptic dopamine is already elevated at baseline.
COMT case study
Genomid has collected several case studies that support the clinical utility of the COMT Val158Met genotype. One example is a 15-yr old male patient with a long history of medication failures. The patient presented chronic progressive depression, amotivation and suicidal thinking. Genetic testing revealed that the patient was Val/Val for the COMT enzyme. Thus, his amotivation and fatigue may have been related to reduced dopamine levels. He was started on dextroamphetamine/amphetamine (e.g., Adderall) to increase synaptic dopamine levels. His symptoms greatly improved, and he demonstrated increased motivation and energy, the ability to engage in extracurricular activities, and cessation of suicidal thinking.
This case is just one example of how COMT genotype results can provide key information to aid in the treatment of your patients.
Learn about the other genes included on the Genomind pharmacogenetic panel, and register to order today.
References
- Stahl, S.M. (2013) Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. 4th Edition, Cambridge University Press, Cambridge.
- Cools, R., & D’Esposito, M. (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biological psychiatry, 69(12), e113–e125. https://doi.org/10.1016/j.biopsych.2011.03.028
- Cools R. Role of Dopamine in the Motivational and Cognitive Control of Behavior. The Neuroscientist. 2008;14(4):381-395. doi:10.1177/1073858408317009
- Sheldrick, A. J., Krug, A., Markov, V., Leube, D., Michel, T. M., Zerres, K., Eggermann, T., & Kircher, T. (2008). Effect of COMT val158met genotype on cognition and personality. European psychiatry : the journal of the Association of European Psychiatrists, 23(6), 385–389. https://doi.org/10.1016/j.eurpsy.2008.05.002
- Frank, M., Fossella, J. Neurogenetics and Pharmacology of Learning, Motivation, and Cognition. Neuropsychopharmacol 36, 133–152 (2011). https://doi.org/10.1038/npp.2010.96
- Lindenmayer, J. P., Khan, A., Lachman, H., McGurk, S. R., Goldring, A., Thanju, A., & Kaushik, S. (2015). COMT genotype and response to cognitive remediation in schizophrenia. Schizophrenia Research, 168(1-2), 279-284. [6512]. https://doi.org/10.1016/j.schres.2015.07.037
- Barnett, J. H., Jones, P. B., Robbins, T. W., & Müller, U. (2007). Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Molecular psychiatry, 12(5), 502–509. https://doi.org/10.1038/sj.mp.4001973
- Poletti, S., Mazza, E., Bollettini, I., Falini, A., Smeraldi, E., Cavallaro, R., & Benedetti, F. (2016). The COMT Val158Met polymorphism moderates the association between cognitive functions and white matter microstructure in schizophrenia. Psychiatric genetics, 26(5), 193–202. https://doi.org/10.1097/YPG.0000000000000130
- Myer, N. M., Boland, J. R., & Faraone, S. V. (2018). Pharmacogenetics predictors of methylphenidate efficacy in childhood ADHD. Molecular psychiatry, 23(9), 1929–1936. https://doi.org/10.1038/mp.2017.234
- Hamidovic, A., Dlugos, A., Palmer, A. A., & de Wit, H. (2010). Catechol-O-methyltransferase val158met genotype modulates sustained attention in both the drug-free state and in response to amphetamine. Psychiatric genetics, 20(3), 85–92. https://doi.org/10.1097/YPG.0b013e32833a1f3c
- Schacht, J. COMT val158met moderation of dopaminergic drug effects on cognitive function: a critical review. Pharmacogenomics J 16, 430–438 (2016). https://doi.org/10.1038/tpj.2016.43
- Cheon, K. A., Jun, J. Y., & Cho, D. Y. (2008). Association of the catechol-O-methyltransferase polymorphism with methylphenidate response in a classroom setting in children with attention-deficit hyperactivity disorder. International clinical psychopharmacology, 23(5), 291–298. https://doi.org/10.1097/YIC.0b013e328306a977
- Mattay, V. S., Goldberg, T. E., Fera, F., Hariri, A. R., Tessitore, A., Egan, M. F., Kolachana, B., Callicott, J. H., & Weinberger, D. R. (2003). Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proceedings of the National Academy of Sciences of the United States of America, 100(10), 6186–6191. https://doi.org/10.1073/pnas.0931309100
- Ma, J., Zhao, M., Zhou, W., Li, M., Huai, C., Shen, L., Wang, T., Wu, H., Zhang, N., Zhang, Z., He, L., & Qin, S. (2021). Association Between the COMT Val158Met Polymorphism and Antipsychotic Efficacy in Schizophrenia: An Updated Meta-Analysis. Current neuropharmacology, 19(10), 1780–1790. https://doi.org/10.2174/1570159X18666201023154049
- Huang, E., Zai, C. C., Lisoway, A., Maciukiewicz, M., Felsky, D., Tiwari, A. K., Bishop, J. R., Ikeda, M., Molero, P., Ortuno, F., Porcelli, S., Samochowiec, J., Mierzejewski, P., Gao, S., Crespo-Facorro, B., Pelayo-Terán, J. M., Kaur, H., Kukreti, R., Meltzer, H. Y., Lieberman, J. A., … Kennedy, J. L. (2016). Catechol-O-Methyltransferase Val158Met Polymorphism and Clinical Response to Antipsychotic Treatment in Schizophrenia and Schizo-Affective Disorder Patients: a Meta-Analysis. The international journal of neuropsychopharmacology, 19(5), pyv132. https://doi.org/10.1093/ijnp/pyv132
- Chen, H., Tu, J., Ni, P., Zhang, W., & Xu, L. (2015). Zhong nan da xue xue bao. Yi xue ban = Journal of Central South University. Medical sciences, 40(6), 623–631. https://doi.org/10.11817/j.issn.1672-7347.2015.06.009
- Rebollo-Mesa, I., Picchioni, M., Shaikh, M., Bramon, E., Murray, R., & Toulopoulou, T. (2011). COMT (Val(158/108)Met) genotype moderates the impact of antipsychotic medication on verbal IQ in twins with schizophrenia. Psychiatric genetics, 21(2), 98–105. https://doi.org/10.1097/YPG.0b013e32834371a7
- Arts, B., Simons, C. J., Drukker, M., & van Os, J. (2013). Antipsychotic medications and cognitive functioning in bipolar disorder: moderating effects of COMT Val108/158 Met genotype. BMC psychiatry, 13, 63. https://doi.org/10.1186/1471-244X-13-63
- Woodward, N. D., Jayathilake, K., & Meltzer, H. Y. (2007). COMT val108/158met genotype, cognitive function, and cognitive improvement with clozapine in schizophrenia. Schizophrenia research, 90(1-3), 86–96. https://doi.org/10.1016/j.schres.2006.10.002
- Weickert, T. W., Goldberg, T. E., Mishara, A., Apud, J. A., Kolachana, B. S., Egan, M. F., & Weinberger, D. R. (2004). Catechol-O-methyltransferase val108/158met genotype predicts working memory response to antipsychotic medications. Biological psychiatry, 56(9), 677–682. https://doi.org/10.1016/j.biopsych.2004.08.012
- Tang, Z., Zhang, S., Guo, D., & Wang, H. (2020). Association between COMT gene Val108/158Met and antidepressive treatment response: A meta-analysis. Gene, 734, 144333. https://doi.org/10.1016/j.gene.2020.144333
- Plewnia, C., Zwissler, B., Längst, I., Maurer, B., Giel, K., & Krüger, R. (2013). Effects of transcranial direct current stimulation (tDCS) on executive functions: influence of COMT Val/Met polymorphism. Cortex; a journal devoted to the study of the nervous system and behavior, 49(7), 1801–1807. https://doi.org/10.1016/j.cortex.2012.11.002
- McClintock, S. M., Martin, D. M., Lisanby, S. H., Alonzo, A., McDonald, W. M., Aaronson, S. T., Husain, M. M., O’Reardon, J. P., Weickert, C. S., Mohan, A., & Loo, C. K. (2020). Neurocognitive effects of transcranial direct current stimulation (tDCS) in unipolar and bipolar depression: Findings from an international randomized controlled trial. Depression and anxiety, 37(3), 261–272. https://doi.org/10.1002/da.22988
- Anttila, S., Huuhka, K., Huuhka, M., Illi, A., Rontu, R., Leinonen, E., & Lehtimäki, T. (2008). Catechol-O-methyltransferase (COMT) polymorphisms predict treatment response in electroconvulsive therapy. The pharmacogenomics journal, 8(2), 113–116. https://doi.org/10.1038/sj.tpj.6500468
- Cho, S. S., & Strafella, A. P. (2009). rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PloS one, 4(8), e6725. https://doi.org/10.1371/journal.pone.0006725
- Slotema, C. W., Blom, J. D., Hoek, H. W., & Sommer, I. E. (2010). Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. The Journal of clinical psychiatry, 71(7), 873–884. https://doi.org/10.4088/JCP.08m04872gre
- Paillère Martinot, M. L., Martinot, J. L., Ringuenet, D., Galinowski, A., Gallarda, T., Bellivier, F., Lefaucheur, J. P., Lemaitre, H., & Artiges, E. (2011). Baseline brain metabolism in resistant depression and response to transcranial magnetic stimulation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 36(13), 2710–2719. https://doi.org/10.1038/npp.2011.161
- Hadley, D., Anderson, B. S., Borckardt, J. J., Arana, A., Li, X., Nahas, Z., & George, M. S. (2011). Safety, tolerability, and effectiveness of high doses of adjunctive daily left prefrontal repetitive transcranial magnetic stimulation for treatment-resistant depression in a clinical setting. The journal of ECT, 27(1), 18–25. https://doi.org/10.1097/YCT.0b013e3181ce1a8c
- Baeken, C., De Raedt, R., Van Hove, C., Clerinx, P., De Mey, J., & Bossuyt, A. (2009). HF-rTMS treatment in medication-resistant melancholic depression: results from 18FDG-PET brain imaging. CNS spectrums, 14(8), 439–448. https://doi.org/10.1017/s1092852900020411
- George, M. S., Taylor, J. J., & Short, E. B. (2013). The expanding evidence base for rTMS treatment of depression. Current opinion in psychiatry, 26(1), 13–18. https://doi.org/10.1097/YCO.0b013e32835ab46d
- Wimber, M., Schott, B. H., Wendler, F., Seidenbecher, C. I., Behnisch, G., Macharadze, T., Bäuml, K-H. T., & Richardson-Klavehn, A. (2011). Prefrontal dopamine and the dynamic control of human long-term memory. Translational Psychiatry, 1(7), e15–e15. https://doi.org/10.1038/tp.2011.15