When choosing a medication to treat your patient’s attention-deficit/hyperactivity disorder (ADHD), there are several genetic biomarkers that may impact the response or side-effect rate of that drug. As discussed in Management of ADHD with a Focus on Pharmacogenetics (PGx) | Pharmacodynamic Genes, pharmacodynamic gene variations can influence an individual’s response to dopaminergic stimulants.
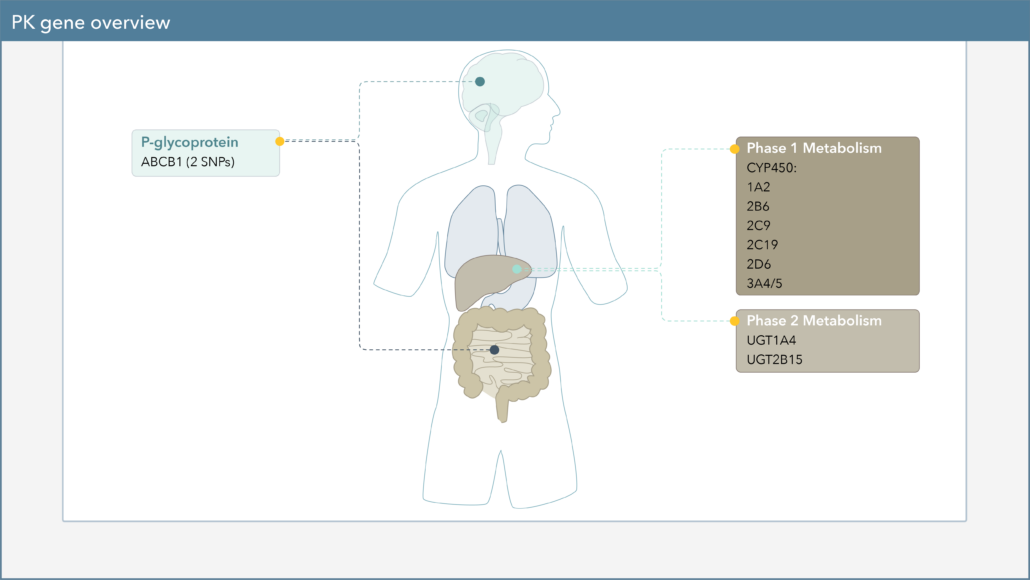
On the Genomind pharmacogenetic (PGx) test, you will also find several pharmacokinetic (PK) genes that can impact various pharmacologic treatment options for ADHD. Physicians can utilize knowledge of an individual’s PK genes to help personalize dosing of these medications and identify patients who may be more likely to experience adverse effects or treatment failure.
How do pharmacokinetic genes affect drug response?
Pharmacokinetics, often described as “what the body does to a drug,” describes a drug’s journey throughout the body and focuses on the absorption, distribution, metabolism, and excretion of drugs.1 One of the major components of PK is drug metabolism, which leads to the biotransformation of drugs within the body. Genetic variation in PK genes can alter drug metabolism, which may influence drug efficacy and safety.
How do CYP450 genetic variations affect drug metabolism?
Cytochrome P450 (CYP450) enzymes are major pathways in drug biotransformation and are responsible for the Phase I metabolism of many medications. In fact, 70-80% of medications are metabolized by the CYP450 system, including several psychotropic medications.2,3 Variations in these genes can lead to alterations in enzyme metabolism. As shown in Figure 1, CYP450 phenotypes are typically reported as extensive/normal (EM/NM), intermediate (IM), poor (PM), rapid (RM), or ultra-rapid metabolizers (UM).
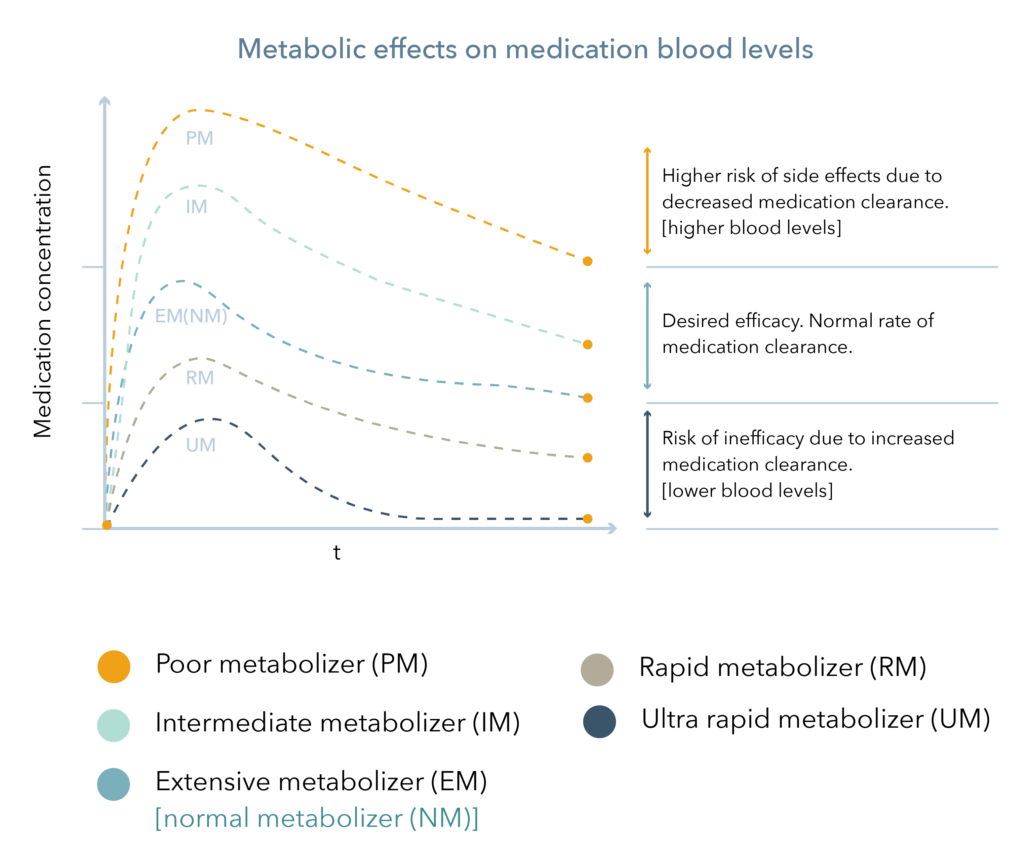
Figure 1
How do pharmacokinetic genes affect ADHD medications?
Variation in CYP450 genes can lead to changes in drug exposure in the following FDA approved medications in ADHD:
- Amphetamine-Dextroamphetamine (Adderall®, Evekeo®)
- Atomoxetine (Strattera®)
- Dextroamphetamine (Dexedrine®, Procentra®, Zenzedi®)
- Guanfacine (Intuniv)
- Lisdexamfetamine (Vyvanse®)
- Viloxazine (Qelbree®)
The package insert for Strattera® (atomoxetine) specifically states that PMs of CYP2D6 have a 10-fold higher area under the curve (AUC) and a 5-fold higher peak concentration to a given dose of Strattera compared with extensive metabolizers. The higher blood levels in PMs leads to a higher rate of some adverse effects of atomoxetine. Dosage adjustments are recommended when administered to CYP2D6 PMs.4
Pharmacogenetic guidance for ADHD medications
Amphetamine, atomoxetine, and viloxazine are included in the FDA Table of Pharmacogenomic Biomarkers in Drug Labeling due to their involvement with CYP2D6. The table includes the following recommendations:5
- Amphetamine: Although the enzymes involved in amphetamine metabolism have not been clearly defined, CYP2D6 is known to be involved with formation of 4-hydroxy-amphetamine. Since CYP2D6 is genetically polymorphic, population variations in amphetamine metabolism are a possibility.
- Atomoxetine: In patients who are known to be CYP2D6 PMs, atomoxetine should be initiated at 0.5 mg/kg/day and only increased to the usual target dose of 1.2 mg/kg/day if symptoms fail to improve after 4 weeks and the initial dose is well tolerated.
- Viloxazine: In a multiple-dose study of healthy volunteers, viloxazine AUC0-24was 26% higher in CYP2D6 PMs compared to EMs.
Additionally, the FDA created a Table of Pharmacogenetics Associations, which includes PGx associations that they have evaluated and for which they believe “there is sufficient scientific evidence to suggest that subgroups of patients with certain genetic variants, or genetic variant-inferred phenotypes are likely to have altered drug metabolism, and in certain cases, differential therapeutic effects, including differences in risks of adverse events”. The Table includes the following recommendations for CYP2D6 PMs:6
- Amphetamine: May affect systemic concentrations and adverse reaction risk. Consider a lower starting dosage or use alternative agents in PMs.
- Atomoxetine: Results in higher systemic concentrations and higher adverse reaction risk. Adjust titration interval and increase dosage if tolerated. Refer to FDA labeling for specific dosing recommendations.
While the FDA guidance regarding these medications only pertains to CYP2D6 PMs, users of PGx testing should also be aware there are other organizations that have reviewed the literature and published PGx guidelines. As discussed in Where Does Evidence for Pharmacogenetics Come From?, the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the Dutch Pharmacogenetics Working Group (DPWG) are excellent resources. For example, CPIC provides guidelines and specific dosing guidance for atomoxetine for both children and adults based on various CYP2D6 phenotypes.7
In order to help clinicians navigate the available PGx guidelines, Genomind provides clinicians with access to Precision Medicine Software. This software serves as a comprehensive tool to help evaluate both gene-drug and drug-drug interactions, PGx guideline recommendations, and alternative medication options, as appropriate. This robust interaction database can help clinicians assess personalized medication options and regimens for their patients. To learn more about the software or to request a demonstration from our PhD and PharmD experts, please utilize Genomind’s Clinician Portal. Not yet registered? Get started today.
Are You Ready to Upgrade Your Practice with Genomind?
Genomind’s PGx testing is the most advanced and comprehensive mental health PGx test available. Get access to 26 genes related to mental health, 130+ medications, 10+ conditions, state-of-the-art tools, and 360 degrees of support.
References
- Zhao M, Wang Y, Chen M, Wu B. Introduction to Pharmacokinetics. In: Circadian Pharmacokinetics. Wu B, Lu, D, Dong D., eds. Singapore: Springer Singapore;2020.
- Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician 2007;76(3):391-396.
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013;138(1):103-41.
- Strattera [package insert]. Eli Lilly and Company. Indianapolis, IN. 2003.
- U.S. Food and Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labeling. Available at: https://www.fda.gov/media/124784/download
- U.S. Food and Drug Administration. Table of Pharmacogenetic Associations. Available at: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations.
- Brown et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Cytochrome P450 (CYP)2D6 Genotype and Atomoxetine Therapy. Clin Pharmacol Ther. 2019 Jul;106(1):94-102