According to the American Psychiatric Association Guidelines for Major Depressive Disorders and the most recent Canadian Network for Mood and Anxiety Treatments (CANMAT), a SSRI, a SNRI, mirtazapine, or bupropion can all be considered as first-line options for the treatment of major depressive disorder (MDD).1-2
Many factors such as clinical features, medication characteristics, and patient factors influence the choice of a first-line antidepressant. SSRIs are the most commonly utilized antidepressants, however, the relative differences between first-line medications are small.3 Selecting an antidepressant involves an individualized assessment of each patient.
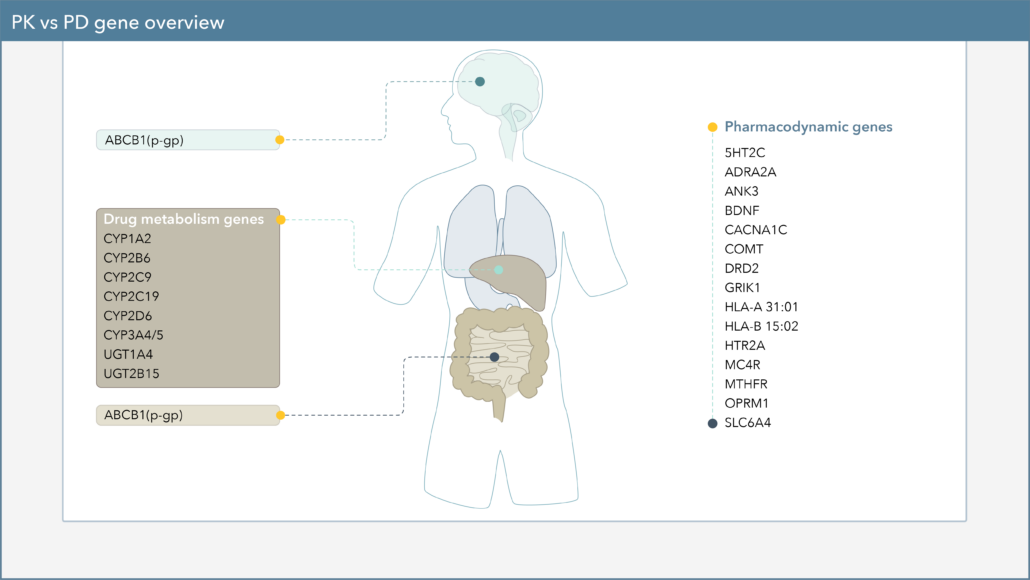
When choosing a medication to treat your patient’s major depressive disorder (MDD), there are several genetic biomarkers that may impact the response or side-effect rate of that drug. On the Genomind pharmacogenetic test you’ll find several pharmacodynamic (PD) genes that can impact medications commonly used for MDD.
How Pharmacodynamic Gene Variation Affects Drug Response
Pharmacodynamics, often described as “what a drug does to the body,” is the study of the biochemical, physiological, and molecular effects of drugs on the body.4 Genetic variations in PD genes could result in changes to these processes and thus affect tolerability and/or likelihood of response to certain drugs.
Let’s discuss a few examples of PD gene variants that may affect first-line pharmacologic treatment options for MDD.
How the Serotonin Transporter Gene (SLC6A4) Variant Affects Antidepressant Response
The landmark STAR*D study demonstrated that the remission rate for MDD after the first trial of an SSRI (i.e. citalopram) is only about 37%, and with every subsequent trial, response rates decrease.5 One PD gene in particular that may be involved in the variability seen in SSRI response is the serotonin transporter gene (SLC6A4).
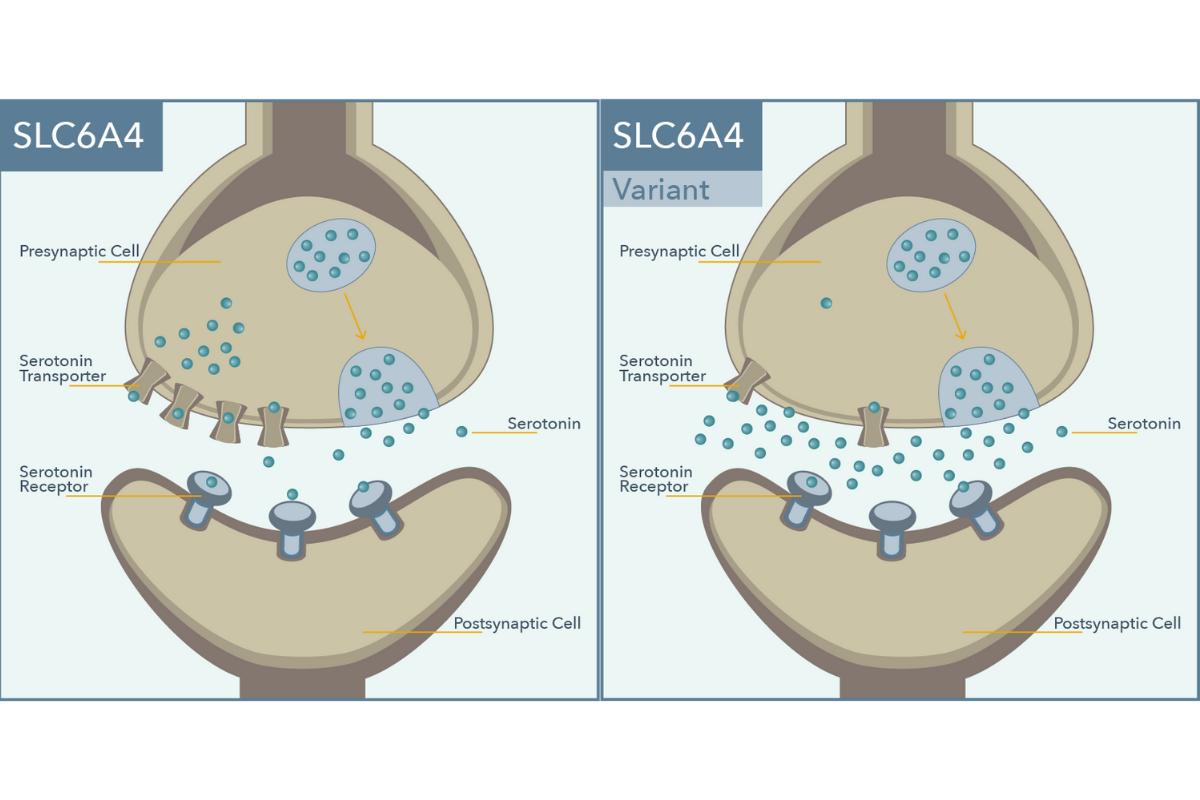
SLC6A4 encodes for the protein responsible for the reuptake of serotonin from synapses. Antidepressant activity of SSRI medications is achieved through inhibition of the serotonin transporter. A polymorphism within the promoter of this gene, i.e. 5-HTTLPR, influences production of the serotonin transporter (SERT), with the long (L) allele associated with twice the basal expression as the short (S) allele.6
A meta-analysis of 6 studies in Caucasian patients with depressive disorders showed that individuals with the S/S genotype were significantly less likely to respond to SSRIs than those with the L/L genotype (OR = 1.71, p = 0.003).6 This polymorphism is also associated with increased risk for side effects in Caucasian S allele carriers.7
How the Brain-Derived Neurotrophic Factor (BDNF) Gene Affects Antidepressant Response
Another PD gene involved with antidepressant response is brain-derived neurotrophic factor gene (BDNF).
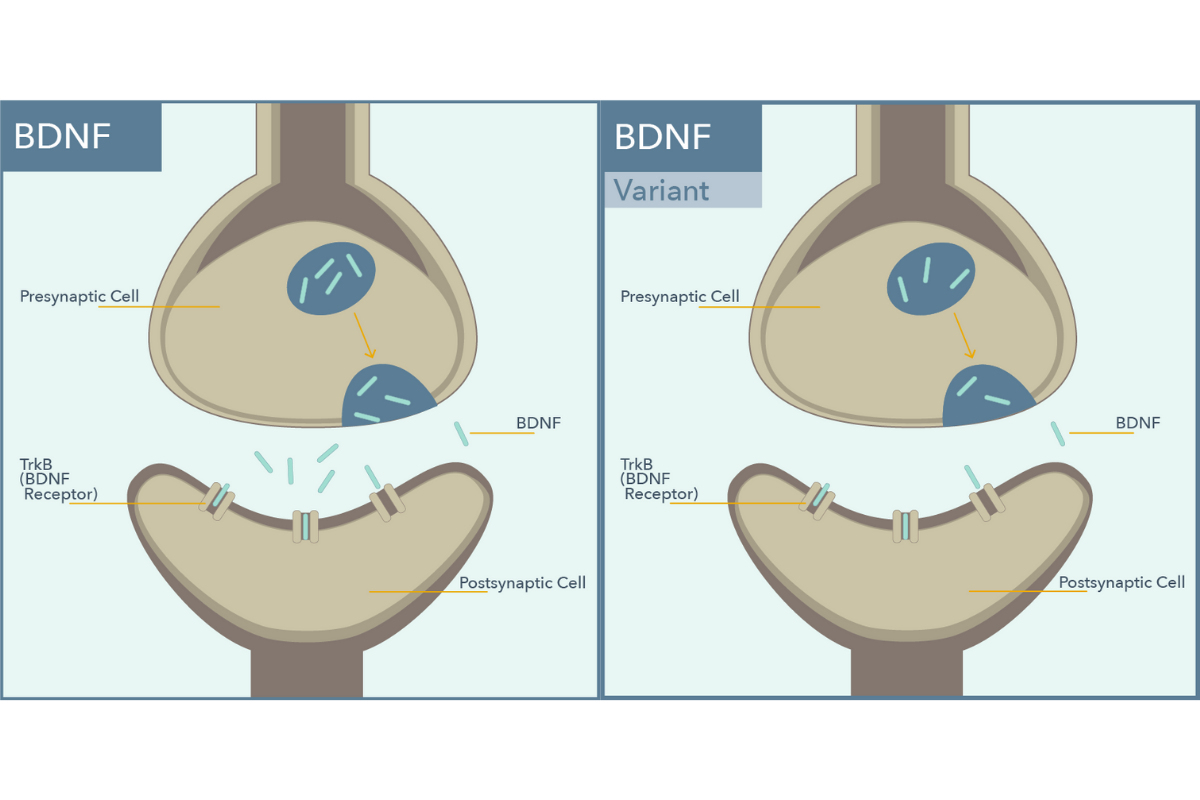
BDNF encodes for a protein that is important in neuronal development and neuroplasticity. Genetic polymorphisms in BDNF have been shown to impact antidepressant response. The Met allele of this gene is associated with decreased activity-dependent BDNF secretion.8
In a 6-month prospective study of 345 Caucasian patients with depression, individuals who had the BDNF Met allele were more likely to have a poorer response to SSRIs and an improved response to SNRIs.9
How the Methylenetetrahydrofolate Reductase (MTHFR) Variant Can Impact Antidepressant Augmentation
Another example of a PD gene that may impact the efficacy of first-line pharmacologic treatment options for MDD is methylenetetrahydrofolate reductase (MTHFR). The MTHFR enzyme is essential for catalyzing the conversion of folic acid to the biologically active form, methylfolate, which is a cofactor needed for serotonin, norepinephrine, and dopamine synthesis.10
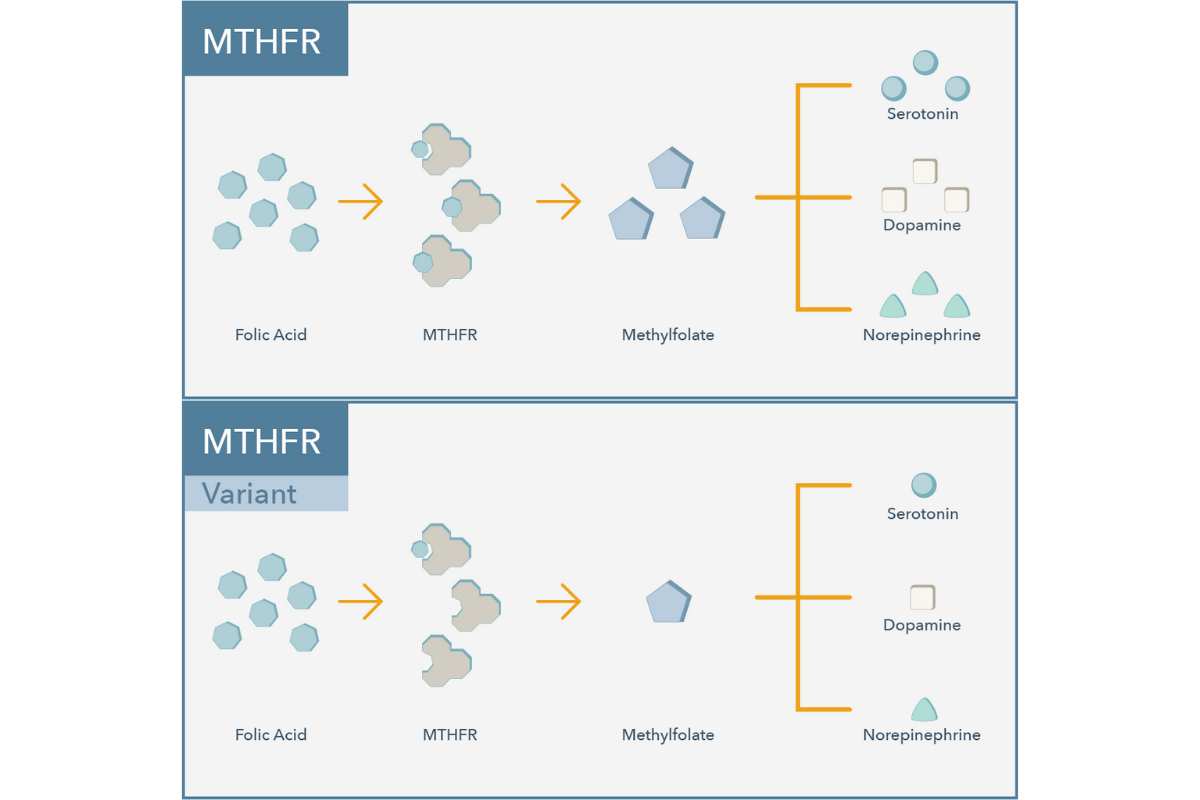
A single nucleotide polymorphism (SNP) in the MTHFR gene (known as the C677T variant) reduces the enzymatic activity of MTHFR by approximately 35% per T allele. Another common SNP, known as A1298C, reduces the enzymatic activity of MTHFR by approximately 20% per C allele. The decrease in enzymatic activity is additive across these polymorphisms.11
MTHFR function is essential for catalyzing the conversion of folic acid to the biologically active form, methylfolate. L‐methylfolate can be taken orally as a supplement, therefore bypassing a dysfunctional MTHFR enzyme. Randomized controlled trials have indicated that L‐methylfolate supplements can be an effective antidepressant augmentation strategy.
For example, Papokastas and colleagues found that treatment of MDD with 15mg of L‐methylfolate in conjunction with an SSRI showed significantly greater efficacy compared to SSRI therapy alone regarding response rate and degree of change in depression symptom scores.12 Another study evaluating a methylfolate B-vitamin complex showed depression remission rates of 42% as monotherapy when MTHFR genotype was taken into consideration.13
In Conclusion
SLC6A4, BDNF, and MTHFR are just a few of the many pharmacodynamic genes that can influence first-line antidepressant response and/or tolerability. Having insight into an individual’s pharmacodynamic genes can offer a more personalized approach that takes into account both the clinical factors and the patient’s genetics.
Are You Ready to Upgrade Your Practice with Genomind?
Genomind’s pharmacogenetic testing is the most advanced and comprehensive mental health pharmacogenetic test available. Get access to 24 genes related to mental health, 130+ medications, 10+ conditions, state-of-the-art tools, and 360 degrees of support. Register today.
References
- Gelenberg AJ, Freeman MP, Markowitz JC, et al. American Psychiatric Association Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Third Edition. Am J Psychiatry 2010;167(suppl):1-152.
- Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder. Can J Psychiatry 2016;61(9):540-560.
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391(10128):1357-1366.
- Karczewski, KJ, Daneshjou R, Altman RB. Chapter 7: Pharmacogenomics. PLoS Comput Biol 2012;8(12):e1002817.
- Rush JA, Trivedi MH, Wisiniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163(11):1905-1917.
- Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol 2012;22(4):239-58.
- Kato M. and Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry 2010;15(5):473-500.
- Martinez-Levy GA, Cruz-Fuentes CS. Genetic and epigenetic regulation of the brain-derived neurotrophic factor in the central nervous system. Yale J Biol Med. 2014;87(2):173-186.
- Colle R, Gressier F, Verstuyft C, et al. Brain-derived neurotrophic factor Val66Met polymorphism and 6-month antidepressant remission in depressed Caucasian patients. J Affect Disord 2015;175:233-40.
- Stahl, S. M. Novel therapeutics for depression: L‐methylfolate as a trimonoamine modulator and antidepressant‐ augmenting agent. CNS spectrums 12, 739‐744 (2007).
- Van der Put, N. M. et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural‐tube defects? Am J Hum Genet 1998;62(5):1044‐1051.
- Papakostas, G. I. et al. Effect of adjunctive L‐methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry 2014;75(8):855‐863.
Mech AW and Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T and A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.